Our portfolio
VMX-C001: Transforming the Treatment of Bleeding in Patients on Factor Xa DOACs
VarmX’s lead compound, VMX-C001, is a modified recombinant human blood clotting Factor Xa that enables normal blood clotting, even in the presence of Factor Xa blood clotting inhibitors.
With its universal and single dosing, ease of use, safety profile, and potential applications in other indications, VMX-C001 is a promising treatment option.
We have recently completed a Phase I study, which marked the first human evaluation of VMX-C001. This investigation not only established the compound’s safety but also demonstrated its successful proof of concept. As part of our strategic timeline, we are planning to commence our pivotal Phase II/III study program in early 2024.
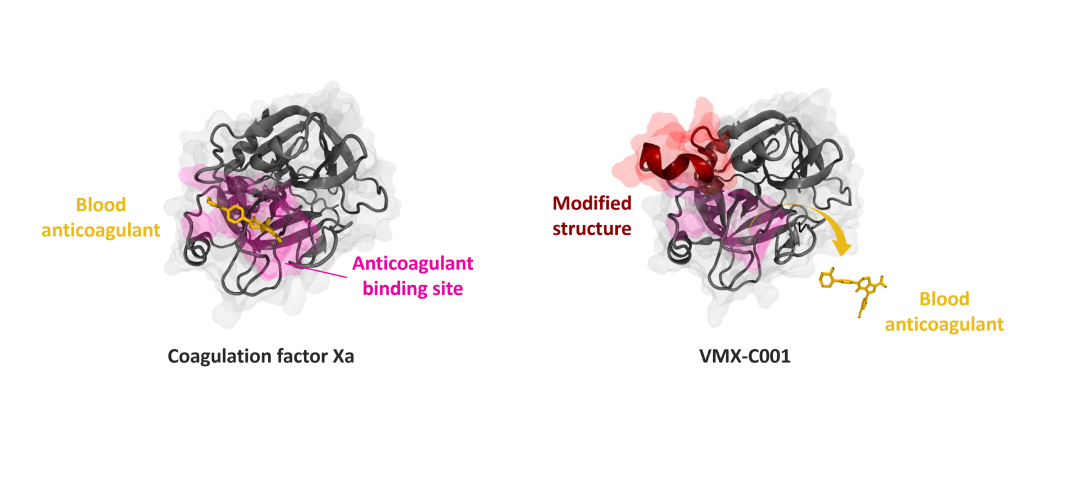
Figure showing the interaction of a blood anticoagulant with normal coagulation factor Xa (left) as opposed to VMX-C001 (right). The protein structure of coagulation factor Xa is shown in grey, and a blood anticoagulant (apixaban) is shown as a stick model in yellow. In factor Xa, anticoagulants bind to the anticoagulant binding site. This region is shown in pink. In VMX-C001, a part of the factor Xa structure is modified (region shown in red). This modification partly obstructs the anticoagulant binding site of factor Xa. As a result, anticoagulant binding to VMX-C001 is hindered, which enables VMX-C001 to clot blood despite the presence of anticoagulants in the blood.
VarmX is committed to advancing therapeutics that can successfully reverse synthetic inhibitors of coagulation and address clotting factor deficiencies.
The aim is to redefine the treatment possibilities within the area of blood coagulation management, offering better outcomes and improved patient care.
The VarmX pipeline program aims to broaden the utility of recombinant factor X as therapeutic protein. Two factor X candidate molecules have progressed to lead candidate phase. One candidate is in development as replacement for plasma-derived factor X additives. The second candidate is in development as second-generation product in which multiple therapeutic applications of factor X are combined into one molecule.
